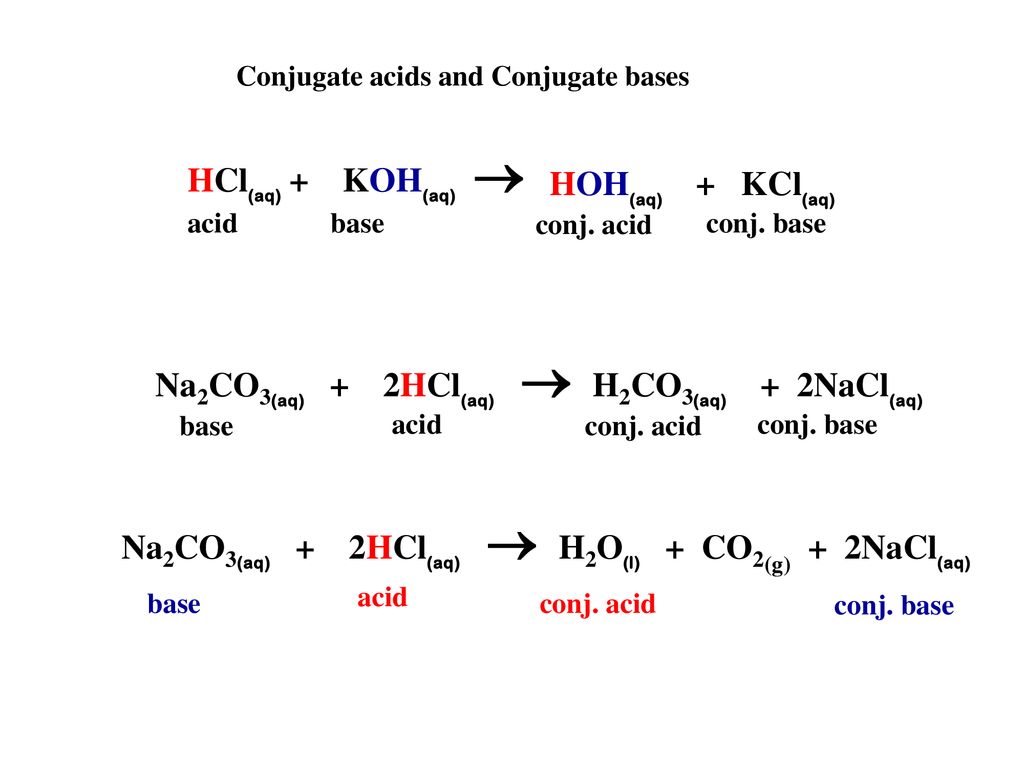
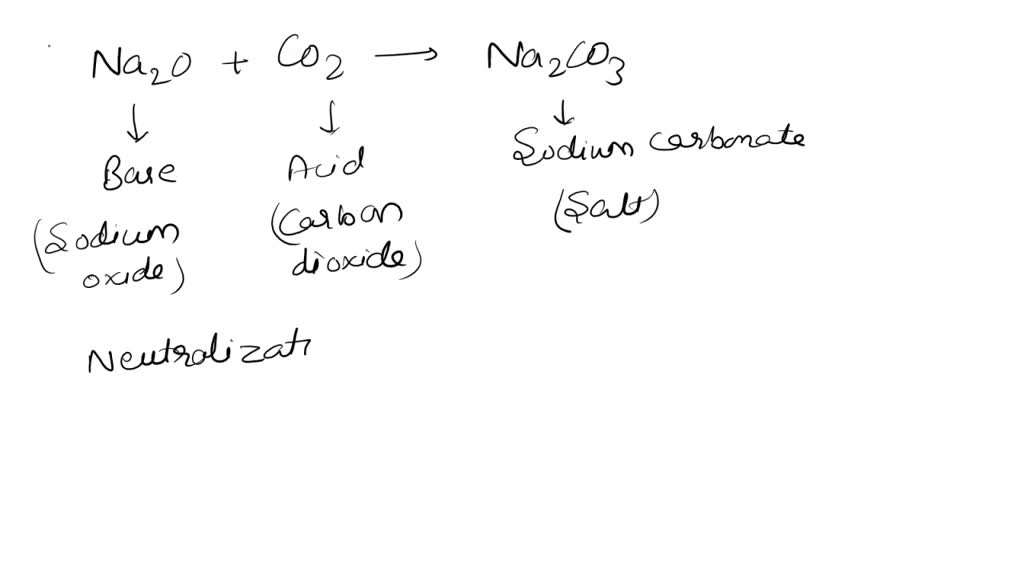
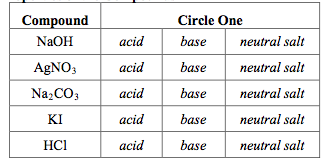
SOLVED: 1. Sodium carbonate is a primary standard base that reacts with hydrochloric acid as follows Na2CO3 + 2 HCl → 2NaCl + H2O + CO2(g) If 40.37 mL of an HCl

Write a mechanism (using curved-arrow notation) for the deprotonation of tannins in base. Use Ar-OH as a generic form of a tannin and use sodium carbonate (Na2CO3) as the base. Balance the
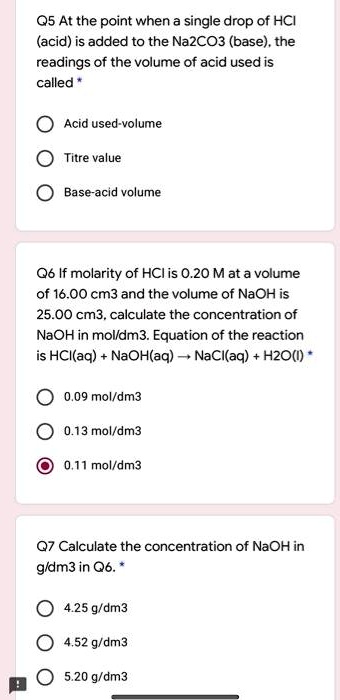
SOLVED: 05 At the point when a single drop of HCI (acid) is added to the Na2CO3 (base) . the readings of the volume of acid used is called Acid used-volume Titre
Explain how a pH meter could be used to find the exact volume of acid required to completely react with a sodium carbonate solution? - Quora

Acid–base titration curves of the biomass pretreated with NaHCO3 sat.... | Download Scientific Diagram

DOC) Standardization of a strong acid (HCl) with a weak base (Na2CO3).docx | Meharaj Ul Mahmmud - Academia.edu
![SOLVED: You may find the Henderson-Hasselbalch equation useful when using buffers [conjugate base] pH pKa log1o [acid] (a) A buffer solution (Buffer 1) contains 0.110 molL-1 sodium hydrogen carbonate (NaHCO3) and 0.052 SOLVED: You may find the Henderson-Hasselbalch equation useful when using buffers [conjugate base] pH pKa log1o [acid] (a) A buffer solution (Buffer 1) contains 0.110 molL-1 sodium hydrogen carbonate (NaHCO3) and 0.052](https://cdn.numerade.com/ask_images/d656061ffc3f4a08a6b158ae3939f3b8.jpg)