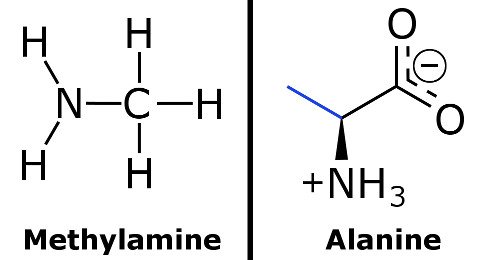
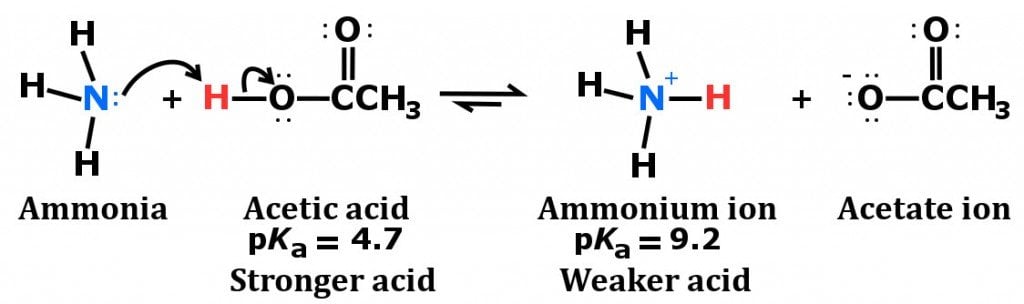
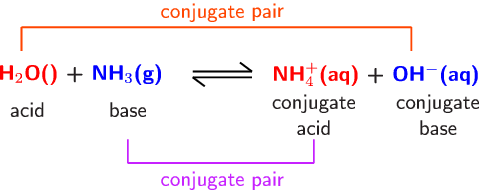
A novel method for molecular transformation to obtain energy from ammonia! | Nature Portfolio Chemistry Community

Spontaneity of the acid–base reaction between acetic acid and ammonia... | Download Scientific Diagram

Question Video: Identifying the Lewis Acid in the Reaction of Ammonia with Boron Trifluoride | Nagwa

Highly efficient and selective separation of ammonia by deep eutectic solvents through cooperative acid-base and strong hydrogen-bond interaction - ScienceDirect

Explain the differences between the Bronsted-Lowry and the Lewis acid-base theories, using the formation of the ammonium ion from ammonia and water to illustrate your points. | Homework.Study.com
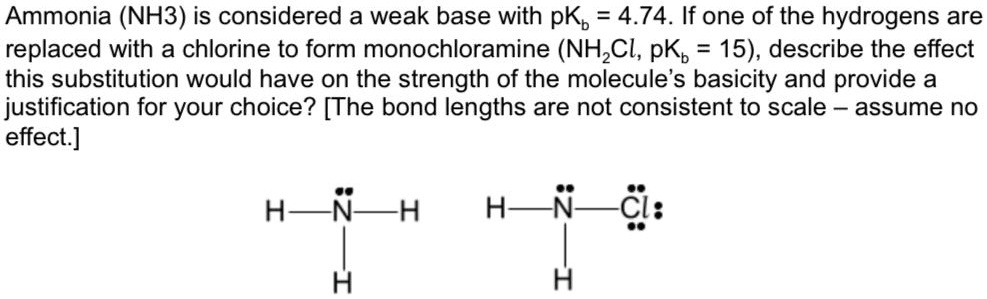
SOLVED: Ammonia (NH3) is considered a weak base with pKb = 4.74. If one of the hydrogens are replaced with a chlorine to form monochloramine (NHZCl, pKb 15) , describe the effect